PrKORSUVA® itch intensity and itch-related QoL data
KORSUVA reduced itch intensity (≥3-point improvement in WI-NRS) at Week 12 from baseline vs. placebo1†
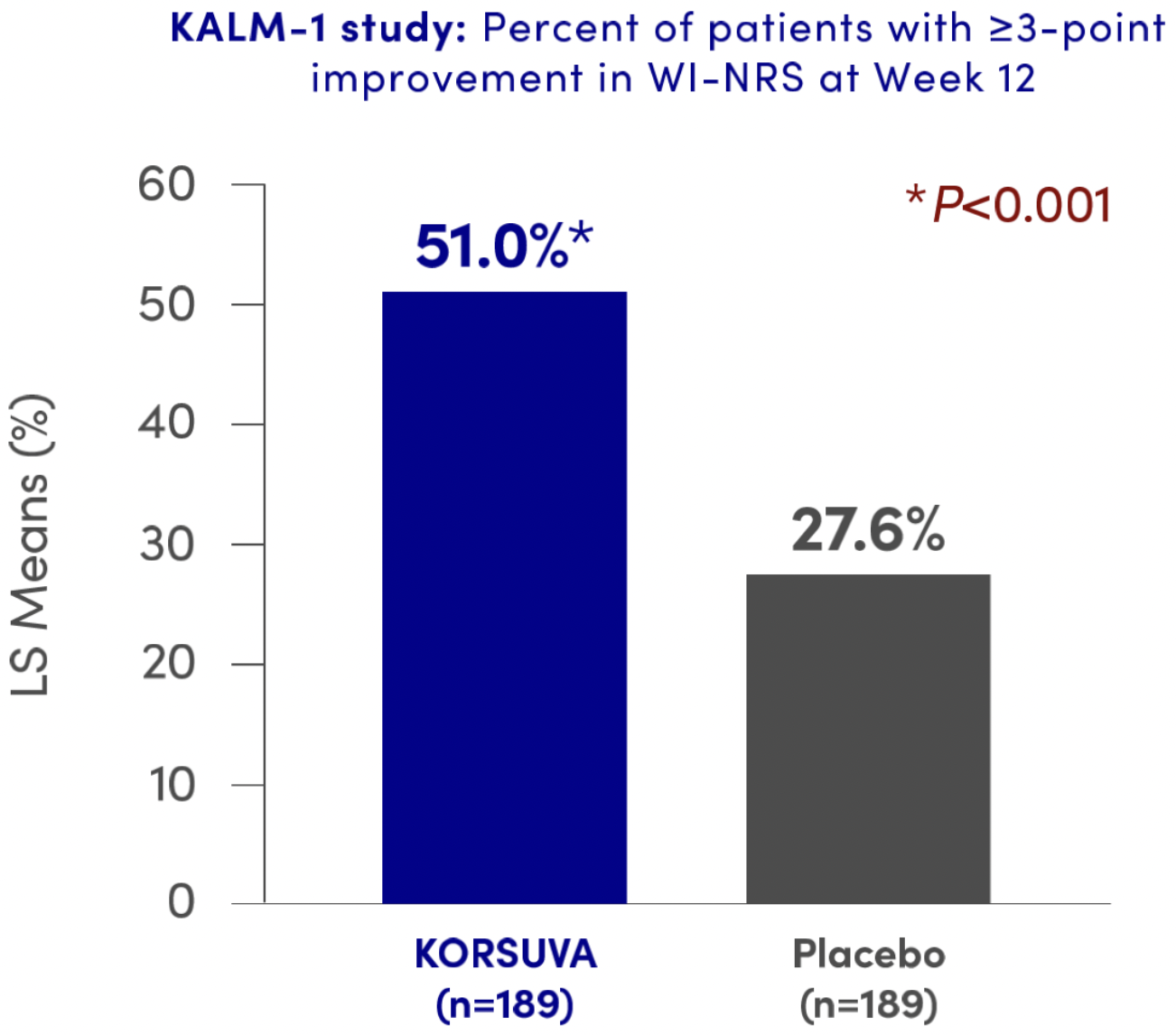
Number observed with ≥3-point NRS improvement: 157 (Yes=82, No=75; missing n=32) and 165 (Yes=51, No=114; missing n=24) for KORSUVA and placebo, respectively. Counts were based on non-missing data.
Estimated percent and P-value used a logistic regression model with terms for treatment group, baseline Worst Itching Intensity numerical rating scale score, use of anti-itch medication during the week prior to randomization, and the presence of specific medical conditions. Missing values were imputed using multiple imputation under missing-at-random missing data assumption for interim patients and post-interim patients separately.
Adapted from the Product Monograph.1
KALM-2 study: 54.0% for KORSUVA vs. 42.2% for placebo (P=0.02; n=237 and 236, respectively. Number observed with ≥3-point NRS improvement: 191 [Yes=95, No=96; missing n=46] and 207 [Yes=77, No=130; missing n=29] for KORSUVA and placebo, respectively. Counts were based on non-missing data.)
Demonstrated results for itch-related QoL (Skindex-10 and 5-D Itch scores) at Week 12 (secondary endpoints)1†
In KALM 2, Skindex-10 and 5-D Itch scores did not reach statistical significance.
KALM-2 study: -16.6 for KORSUVA vs. -14.8 for placebo (P=0.171; n=237 and 236, respectively)
KALM-2 study: -4.9 for KORSUVA vs. -3.8 for placebo (n=237 and 236, respectively; not tested based on the hierarchical testing order, as the prior secondary endpoint [total Skindex-10 Scale score at Week 12] was not statistically significant.)
Adapted from the Product Monograph.1
† KALM-1 and KALM-2 studies: Two randomized, multicentre, double-blind, placebo-controlled trials that enrolled a total of 851 subjects 18 years of age and older undergoing hemodialysis who had moderate-to-severe pruritus. KALM-1 was a US study, and KALM-2 was a global study. Subjects received either placebo or 0.5 mcg/kg KORSUVA intravenously 3 times a week following hemodialysis for 12 weeks. Patients were permitted to continue with the use of anti-pruritic medications (e.g., antihistamines, corticosteroids, gabapentinoids). Both studies included a double-blind phase and an open-label phase (52 weeks).

