PrKORSUVA® has a well-established safety profile1
Treatment-emergent adverse reactions reported in ≥2% in the KORSUVA group or ≥1% higher than placebo group in pooled analyses for the 12-week placebo-controlled periods of the phase 3 trials (KALM-1 and KALM-2) regardless of causality determined by the study investigators.
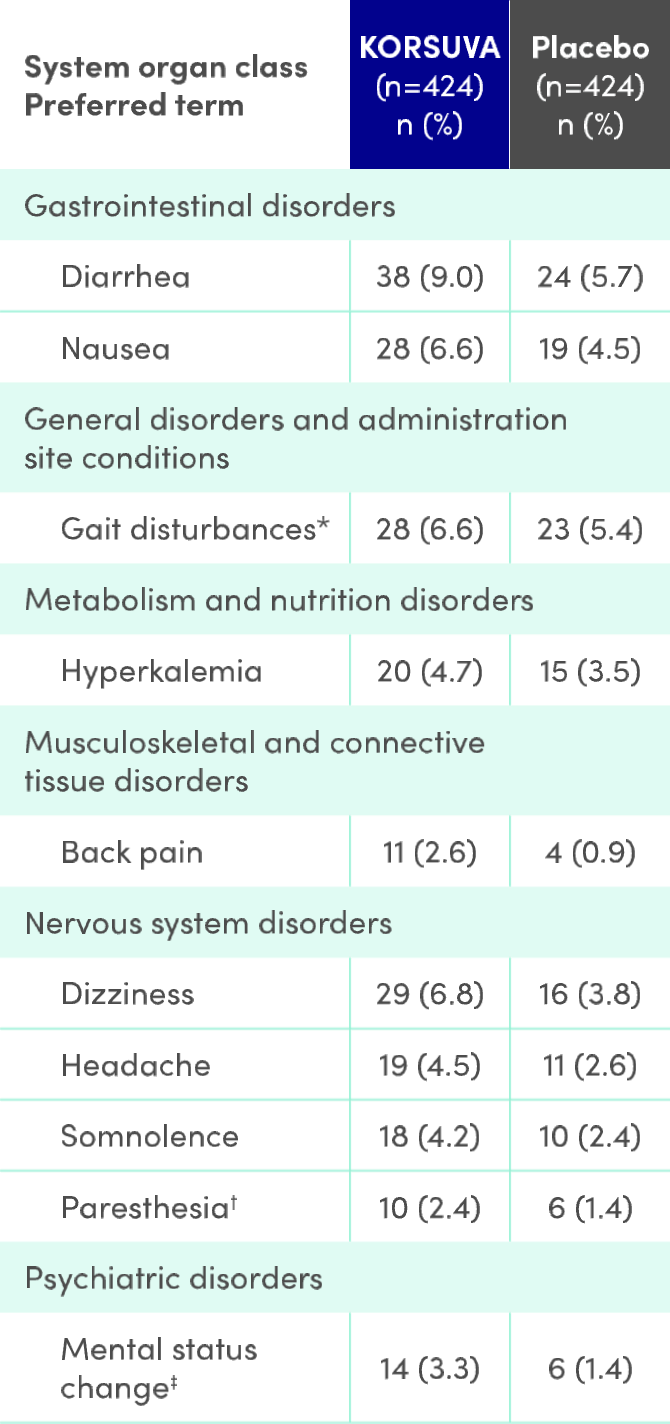
- Adapted from the Product Monograph.1
- * Gait disturbances includes: preferred terms of falls and gait disturbances.
- † Paresthesia includes: preferred terms of paresthesia, hypoesthesia, paresthesia oral and hypoesthesia oral. A patient was counted only once for each preferred term if multiple events of the same preferred term were reported for the same patient.
- ‡ Mental Status Change includes: preferred terms of confusional state and mental status change.
- In the 52-week open-label extension periods of the trials, there were no additional safety issues identified.

